What Companies Should Know About How FDA is Regulating Hand Sanitizers During COVID-19
Hand sanitizers are generally regulated by the U.S. Food and Drug Administration (FDA) as Over-the-Counter (OTC) drug products. However, during the coronavirus (COVID-19) pandemic, consumers and health care professionals have experienced difficulties in sourcing alcohol-based hand sanitizers such that FDA has relaxed certain requirements on a temporary basis to facilitate the availability of these important products.
During the COVID-19 pandemic public health emergency, the FDA Center for Drug Evaluation and Research (CDER) has issued important, temporary policies to aid companies in the compounding, preparation, and distribution of hand sanitizer products for consumer and health care use. These policies are:
- The Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry (the Temporary Preparation Policy);
- The Temporary Policy for Manufacture of Alcohol for Incorporation into Alcohol Based Hand Sanitizer Products During the Public Health Emergency (COVID-19); and
- The Policy for Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency.
This overview is specifically designed to focus on explaining the Temporary Preparation Policy for ethanol-based hand sanitizer products. Please note that these FDA policies also apply to the preparation and manufacture of hand sanitizer using isopropyl alcohol. However, the temporary policies are not applicable to wipes or sprays or antibacterial soaps.
Who can prepare and distribute a hand sanitizer under the Temporary Preparation Policy?
The Temporary Preparation Policy applies to “firms” or companies that undergo an expedited registration process as OTC drug manufacturers by registering their facilities and listing the alcohol-based hand sanitizers they plan to produce in the FDA Drug Registration and Listing System. Once registered, companies receive automatic confirmation from the FDA and may begin to manufacture and distribute compliant hand sanitizer immediately.
Who can manufacture ethanol for use in a hand sanitizer under the Temporary Manufacture Policy?
As with entities seeking to prepare and distribute hand sanitizer, the Temporary Manufacture Policy applies to “firms” or companies that undergo an expedited registration process by registering their facilities and listing the alcohol they plan to manufacture in the FDA Drug Registration and Listing System. Once registered, companies receive automatic confirmation from the FDA and may begin to manufacture ethanol for use as the Active Pharmaceutical Ingredient (API) in alcohol-based hand sanitizers for consumer use and for use as health care personnel hand rubs for the duration of the public health emergency declared by the Secretary of the U.S. Department of Health and Human Services (HHS). Ethanol made to food grade specifications or by distilleries may be used in these products. As discussed further in this memorandum, ethanol produced in facilities normally producing fuel or technical grade ethanol may be considered for use if the ethanol is produced from fermentation and distillation as would be typically used for consumable goods, and no other additives or other chemicals have been added to the ethanol.
What formula must be used pursuant to the Temporary Preparation Policy?
The FDA’s temporary policies facilitate the use of the World Health Organization (WHO) harmonized formula for hand sanitizers. According to the Temporary Preparation Policy, qualifying ethanol-based hand sanitizers must be manufactured using the following formulation and ingredients:
- Alcohol (ethanol) (Chemical Abstract Services Registry Number (CASRN) 64-17-5) (also called ethyl alcohol) 80%, volume/volume (v/v) in an aqueous solution that is:
- Not less than 94.9% ethanol by volume. (Lower ethanol content alcohol may be used as long as the finished hand sanitizer meets the ethanol volume to content concentration of 80%.)
- Derived from distillation or fermentation processes typically used for consumable goods, OR if derived from synthetic processes, ethanol that is United States Pharmacopoeia (USP) or Food Chemical Codex (FCC) “food grade.”
- Denatured according to Alcohol and Tobacco Tax and Trade Bureau (TTB) regulations in 27 CFR Parts 20 and 21. More information of the Policy’s denaturing requirements is set out below.
- Glycerol/Glycerin (1.45% v/v) that is:
- Hydrogen peroxide (0.125% v/v) that is:
- Sterile water that is:
- Boiled, distilled, or otherwise processed in a way that results in water that meets the specifications for Purified Water USP. Water sterilized using micropore filtration membrane systems consistent with microbial removal will also be considered (0.45 mesh was identified as suitable during the March 30, 2020 stakeholder call).
- Used as quickly as possible after it is rendered sterile or purified.
Companies cannot add any other active or inactive ingredients to their hand sanitizer other than those specified above. The Temporary Preparation and Manufacture Policies require the exclusive use of these exact ingredients.
FDA cautions that ingredients that are described as only meeting American Chemical Society (ACS) grade standards should generally not be used in hand sanitizers. (The ACS standards for reagents are not designed to determine the suitability of a chemical for human use). However, if you have concentration and impurity information, FDA is willing to make case-by-case recommendations.
Similarly, technical grade Isopropyl Alcohol should not be used as an active ingredient but may be used as a denaturant. Companies can use concentrate USP, topical grade, or tech grade hydrogen peroxide as long as the hand sanitizer formula is adjusted based on what grade is being used to ensure the active ingredient concentration remains consistent.
In terms of related observations, the FDA Temporary Preparation Policy specifies the use of 80% alcohol in alignment with WHO recommendations – even though FDA’s 1994 Temporary Final Monograph (TFM) specifies that alcohol may be used in a final product concentration between 60-95% (v/v). Also, TTB guidance on the production of hand sanitizer during the COVID-19 pandemic recognizes that FDA requires the exclusive use of denatured ethanol and addresses additional relief from TTB requirements.
What sanitation conditions are required by the Temporary Preparation Policy? Is compliance with current Good Manufacturing Practices (cGMPs) required?
Unlike similar FDA guidance directed at pharmacies and other FDA registered compounders, the Temporary Preparation Policy does not require strict adherence with current Good Manufacturing Practices (cGMPs). Instead, the Temporary Preparation Policy requires that “hand sanitizer is prepared under sanitary conditions and equipment utilized is well maintained and fit for this purpose.” It further explains that in alignment with 21 U.S.C. § 351(a)(2)(A), facilities must prevent preparing, packing, or holding the hand sanitizer under insanitary conditions whereby it may be contaminated with filth, or whereby it may have been rendered injurious to health. Therefore, while companies may look to cGMPs for guidance, it is possible for firms to determine that their conditions are suitable, sanitary, well-maintained, and fit for the purpose of making hand sanitizer even if they do not have all cGMP requirements currently in place. That being said, because the Temporary Preparation Policy is temporary, companies who wish to continue producing hand sanitizer after the COVID-19 pandemic will need to meet the cGMP conditions down the road.
What other requirements apply to the manufacture of ethanol and preparation and distribution of hand sanitizers under the FDA policies?
In addition to meeting the formula and sanitation requirements set out above, the following conditions apply to ethanol manufacturing facilities for supplying raw material for incorporation into hand sanitizers:
- Denaturing Method. The ethanol must be denatured either by the alcohol producer or at the point of production of the finished hand sanitizer product. TTB regulations in 27 CFR Parts 20 and 21 provide a number of formulas for denaturing alcohol. However, the Temporary Manufacture Policy lists preferred methods in Appendix C, including Formula 40A or 40B with or without the tert-butyl alcohol. In addition, FDA is considering additional denaturants that have been suggested by industry and will evaluate these on a case-by-case basis. To submit a potential denaturant for review please contact the FDA directly through this email address. As more denaturants become available, the FDA will continue to update its guidance document.
- Testing. If the alcohol is to be distributed to another firm for producing the hand sanitizer, it must be labeled with the ethanol content determined by an appropriate test so that the hand sanitizer can be reliably produced at the intended strength. The alcohol production firm needs to use the most accurate method of analysis available at the site for verification of ethanol content in a sample. Methods can include gas chromatography (GC), specific gravity (e.g., alcoholmeter, hydrometer, pycnometer, or gravity density meter), or another test that is at least as accurate. The sample tested can be from the final API before packaging (if distributed as an API) or before actual use in producing the hand sanitizer. A simple record should be used to document key steps and controls.
- Labeling. The alcohol API, if distributed to other producers, must be labeled in alignment with the examples specified in the Temporary Manufacture Policy.
- Recordkeeping. Firms must keep a record to document key steps and controls that assure each batch of hand sanitizer matches the formula developed for the drug product. FDA expects companies to use the most accurate method of analysis available at the site (including gas chromatography (GC), alcoholmeter, hydrometer, or other chemical analysis of equivalent accuracy) to verify the alcohol content in samples of the finished hand sanitizer before each batch is released for distribution. Firms must also label their hand sanitizer consistent with the examples specified in the appendices to the Temporary Preparation Policy.
In addition, containers used for hand sanitizers should seal sufficiently (appropriate for liquids) to keep the alcohol from evaporating, and not degrade.
Can fuel manufacturers supply ethanol into the hand sanitizer market?
Yes. On a stakeholder call hosted by the FDA on March 30, 2020, FDA recommended that fuel ethanol processes be reviewed on a case-by-case basis. FDA offered to participate in consultations and review technical specification sheets with individual companies for this purpose. The Agency’s main concern is the addition and presence of potential contaminants. The Agency informed participants that if manufactured ethanol is acquired from traditional corn-based fermentation or distillation processes, it is likely to meet the USP/FCC standards. In case of questions or queries with regards to the feasibility of usage, FDA encouraged interested parties to reach out directly using the FDA’s COVID-19 email address.
What considerations apply with respect to impurities?
Special caution should be taken to ensure any other chemicals on site are not introduced into ethanol either intentionally or via cross contamination. Ethanol should be screened for potentially harmful impurities, including those specified in the USP and FCC requirements. Companies should seek technical guidance from the FDA directly.
On Thursday, April 16, 2020, FDA held two conference calls to discuss updates and clarifications to the Agency’s recent guidance materials on the production of hand sanitizer during the COVID-19 pandemic. The first call was directed at stakeholders producing fuel ethanol and the second call was directed at all other stakeholders. Both calls focused on the need to be mindful of contaminants (introduced into hand sanitizers either intentionally or via cross contamination) and highlighted EPA’s willingness to conduct tox analyses for individual products.
On June 1, 2020, FDA updated this policy with respect to requirements for fuel and technical grade ethanol, in order to specify interim levels of certain impurities that FDA has determined can be tolerated for a relatively short period of time, given the emphasis on hand hygiene during the COVID-19 public health emergency. In its update, FDA includes as Attachment 1 to the Temporary Policy clarifying guidance concerning fuel or technical grade ethanol that does not meet USP or FCC requirements. FDA’s Temporary Policy now states that this source of ethanol “may be considered for use” when the following circumstances are present:
- Fuel or technical grade ethanol does not contain gasoline or any of its components (e.g., n-heptane).
- The following impurities meet the interim limits listed in Table 1 below (NMT = Not More Than) and no other potentially harmful impurities are present other than those addressed in Table 1. The asterisk in Table 1 refers readers to further observations on acetaldehyde which can be reviewed in detail in the Guidance at the link provided above.
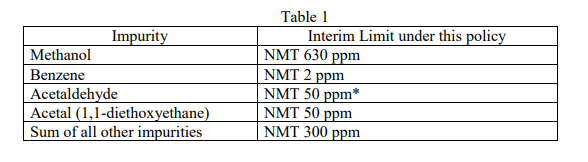
In cases where fuel or technical grade ethanol that does not meet the interim limits in Table 1 because the sum of all other impurities exceeds the interim limit of 300 ppm, the ethanol still will be acceptable for use if all individual impurities are identified and meet the interim limits in Table 2 below.
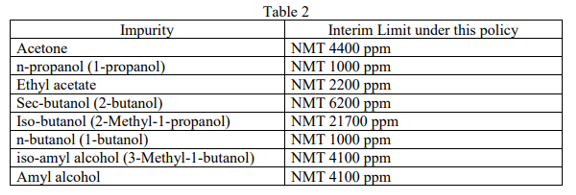
Finally, for any impurity not listed in Table 1 or Table 2, firms must submit data with the level for each individual impurity with information regarding the safety of each impurity to COVID-19-Hand-Sanitizers@fda.hhs.gov with “ETHANOL DATA” in the subject line for FDA’s assessment regarding the use of the ethanol under this policy.
When does denaturing of the ethanol used in hand sanitizers under the Temporary Manufacture Policy have to occur?
Calls to poison control centers related to hand sanitizer consumption have been on the rise, so there is currently no flexibility on the addition of denaturants in hand sanitizers. However, there is flexibility on where and when the denaturants are added (as long as it is done prior to sale). The alcohol may be denatured at the point of production by the alcohol production firm or the point of manufacture or compounding of the hand sanitizer. However, the alcohol intended for incorporation into a finished product must be labeled accurately as “denatured” or “undenatured.” Note that the Temporary Manufacture Policy emphasizes that beyond alcohol, water, and denaturants (if added at the point of production), alcohol production firms must not add other ingredients to the API.
Does a hand sanitizer need a Safety Data Sheet (SDS) and is there assistance to prepare one?
To manufacture and ship hand sanitizers, businesses need to prepare an SDS. The Underwriters Laboratory (UL) has created an SDS for the ethanol-based hand sanitizer formula that complies with WHO recommendations and FDA’s temporary requirements. The SDSs are available in the relevant regional Globally Harmonized System (GHS) formats free of charge.
Are there rules with respect to transporting hand sanitizers?
Hazardous materials regulations (or HMR, 49 CFR Parts 171-180) apply to transporting hand sanitizers because they are typically classified according to the HMR as a Class 3, Flammable Liquid. The Pipeline and Hazardous Materials Safety Administration (PHMSA) has temporarily eased some requirements for transporting alcohol-based hand sanitizers on highways. If offered for transportation in the quantities and packaging that are specified in § 173.150(g), hand sanitizers are excepted from other HMR requirements. PHMSA is providing enforcement discretion for additional packaging configurations and sizes to facilitate transportation of these vital commodities from facilities operating under the FDA’s Temporary Policies. Companies still must follow federal requirements for packaging, labeling, and training. Information on DOT (and Transport Canada’s) requirements for the transportation of hand sanitizers can be found here.
Is Adverse Effects Reporting required under the Temporary Preparation and Manufacturing Policies?
According to the Temporary Preparation and Manufacture Policies, firms preparing hand sanitizers will need to have a way to accept adverse event reports for any products they manufacture and must submit adverse event reports to FDA. For more information, please see FDA’s guidance on adverse event reporting requirements. Separately, if alcohol production firms receive adverse event reports, they are encouraged to submit them to FDA’s MedWatch Adverse Event Reporting program.
How long will the Temporary Preparation and Manufacturing Policies be in place?
FDA has stated that when the public health emergency is over, as declared by the Secretary of HHS, it intends to discontinue its enforcement discretion policies and withdraw its guidance. Public health emergency declarations must be periodically renewed but there is no overall time limit on them. For example, the public health emergency on opioid use has been in effect for several years. Should FDA find at some point in the future that there is no concern with product shortages, it may lift the policy earlier, but notice to the public would be provided in advance.
On Thursday, June 11, 2020, Senator Thune (R-SD) introduced the Hand Sanitizer Guidance Extension Act of 2020. This bill seeks to provide at least two additional years of certainty concerning FDA’s temporary guidance from the date of enactment. The legislation would see FDA’s Temporary Policy extended for an additional year if the active health emergency is still in effect when the extension would otherwise expire. In tangible terms, this bill would provide hand sanitizer makers flexibility in not requiring drug establishment registration and cGMP certification for their facilities while functioning under this temporary guidance. It also provides some measure of certainty for those manufacturers who may have had to make CAPEX investments to get their product lines ready for FCC/USP grade EtOH production. Recognizing there is ongoing deliberation with the FDA about denaturants and accepted impurities, this legislation provides a baseline of certainty while still allowing both case-by-case approvals from FDA and for FDA to waive or reduce other requirements as necessary to meet the public health emergency.
What if I have additional questions regarding production and availability of these products?
FDA has provided an email address for process and raw material related queries. Helpful resources include USP’s Hand Sanitizer Toolkit. In addition, the Personal Care Products Council (PCPC) has launched an online platform to connect buyers and sellers of hand sanitizer ingredients and packaging materials. PCPC is designing the online platform as a public service, free of charge for participants. The program will be open to non-members and will offer participants the ability to search according to their specific business needs and receive a list of other participants who can match those needs (by commodity type, quantity, and recent availability).
For more information, questions, comments, or assistance, please do not hesitate to contact Martha Marrapese (mmarrapese@wiley.law or 202-719-7156) or Tracy Heinzman (theinzman@wiley.law or 202-719-7106).


